Is high efficiency filter leakage rate test: ≤0.01% necessary?
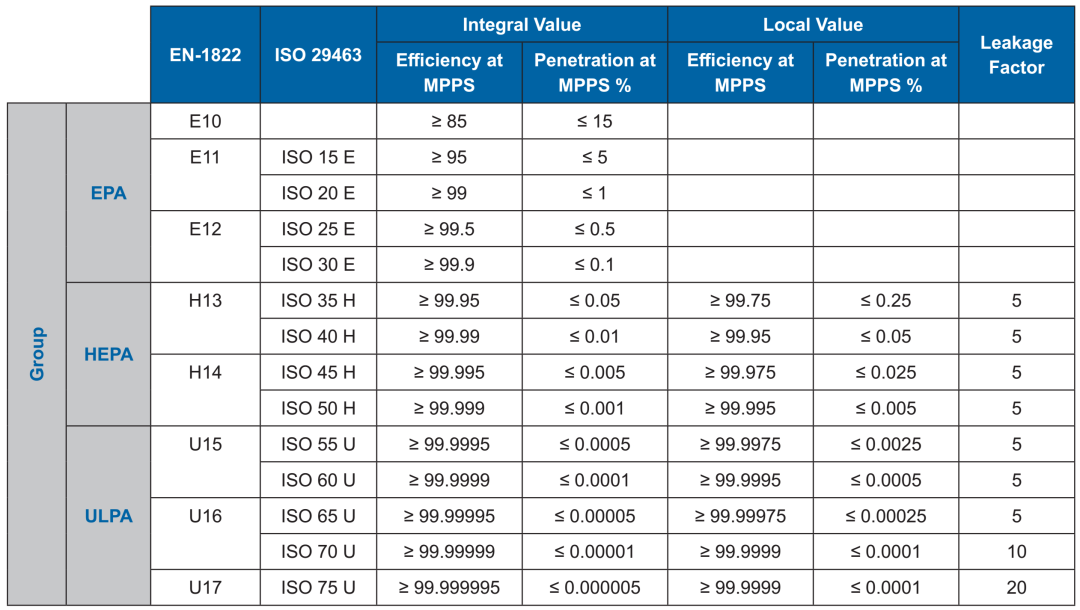
What is the acceptance criterion for HEPA filters?
Most test standards regarding the acceptance criterion for HEPA filter leak rates state that the acceptable leak limit is ultimately determined by the customer and supplier. However, for many applications using HEPA filters or different levels of cleanroom, most adopt a scan test leak criterion of ≤0.01%. Although 0.01% leak rates have been used historically and their origins are related to the accuracy of early photometer test equipment, using a 0.01% leak rate criterion as the acceptance criterion without a scientific and risk-based assessment will lead to problems associated with leak testing and may result in significant operating costs if an over-limit or failure is found in a low-risk area. Filters are not 100% retaining and particles near the MPPS are expected to penetrate the filter partially or entirely. When using lower-level HEPA filters, the factory-performed acceptable leak rate criterion for particles at or near the MPPS may be equal to or greater than the acceptance criterion for field leak rate testing, and testing acceptance criteria becomes more controversial and difficult. This is especially true in areas where leaks may occur. Therefore, when purchasing filters, it is important to consider the filter rating and how it will be tested after installation to avoid unnecessary field test failures.
ISO 14644-3 [33] provides guidance on how to implement alternative leakage criteria. In a risk-based approach, the ideal acceptance criterion is one that reflects the efficiency of the filter used or the cleanliness of the room being tested. ISO 14644-3 uses the factory filter efficiency rating as the basis for negotiating the acceptance criterion. The leakage acceptance criteria for photometric leak testing and particle counter-based leak testing should be the same because the theory and methodology behind both methods are the same. If performed properly, leak tests using a photometer and a particle counter will give the same leak rate results (Meek et al., 2011 [121]).
If the detected leakage exceeds 0.01% of the upstream concentration, it is generally considered that the leakage rate exceeds the maximum permissible standard.
However, for filter systems with an overall efficiency MPPS ≥ 99.95% and less than 99,995% (such as H13 filters), the acceptance standard is 0.1%. If a filter system with an overall efficiency of less than 99.95% MPPS is to be tested, a different acceptance standard is required, depending on the agreement between the customer and the supplier.
Importance of High Efficiency Particulate Air Filters (HEPA) and Leak Detection Methods and Standards
Cleanrooms require air filters to prevent contaminants from entering the cleanroom through their HVAC systems. Cleanrooms are controlled environments where the control of temperature/humidity, pressure, and particulate matter is critical for optimal operational performance. Cleanrooms are designed based on the cleanliness required for a specific process. In the semiconductor industry where microchip manufacturing is performed, air filtration has a higher efficiency because microchips are very sensitive to particles, especially small particles that get wedged between the conductive circuits on the wafers when the wafers are handled.
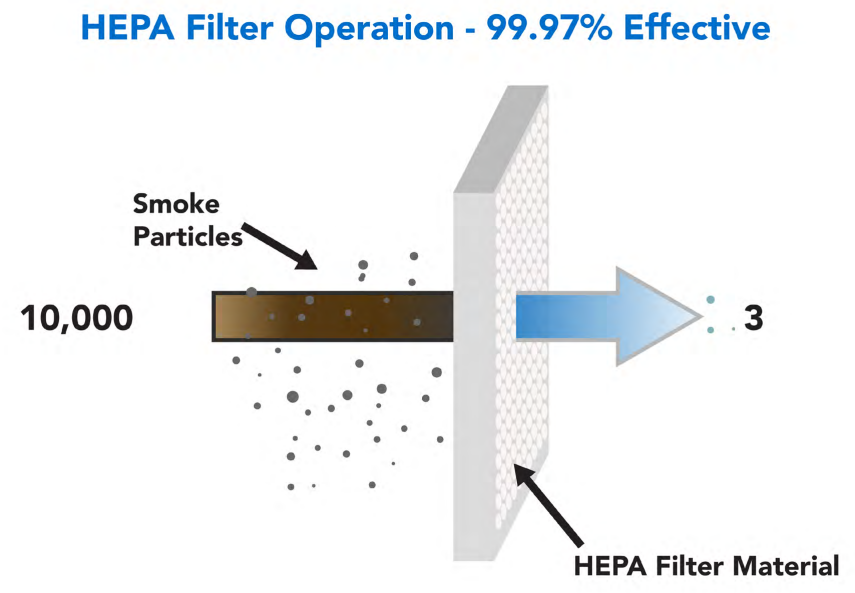
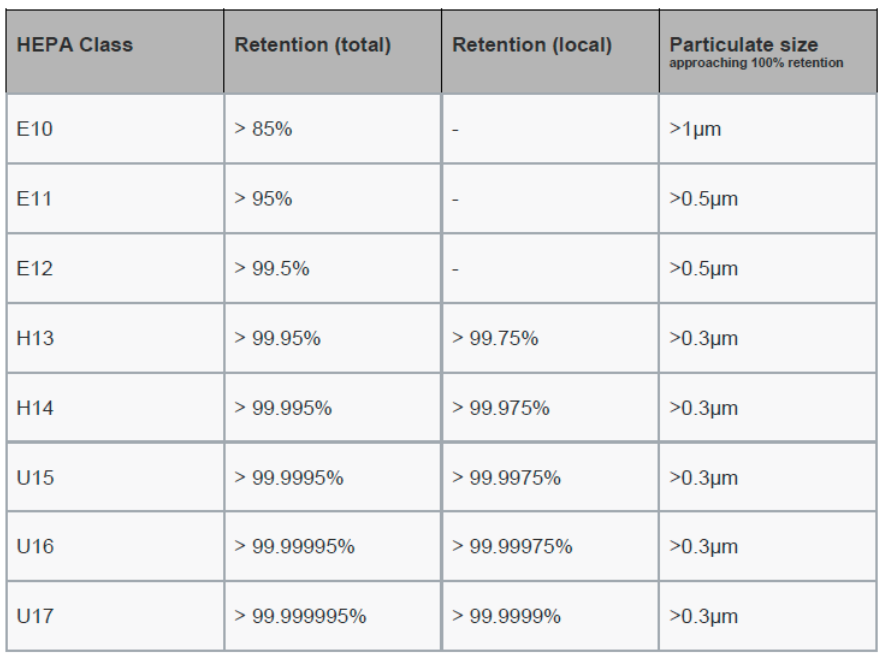
High Efficiency Particulate Air Filters (HEPA), these filters are very effective in contamination control. They filter particles as small as 0.3 microns and are widely used in pharmaceutical facilities. ULPA (Ultra High Particulate Air Filters) filter particles below 0.12 microns and are widely used in semiconductor equipment.
Importance of Filters in Cleanrooms
Managing Contamination Control in Cleanrooms through Several Activities High Efficiency Particulate Air Filters or UPLA Filters play a vital role in cleanrooms. High Efficiency Particulate Air (HEPA) filters must be as efficient as 99.97% penetrating particles, 0.3 microns. This efficiency rating is given by the U.S. Department of Energy to qualify as a true HEPA filter.
HEPA Filter Specifications
As defined by the U.S. Department of Energy (DOE) standards adopted by most U.S. industries, the minimum resistance or pressure drop a HEPA filter presents to airflow is usually specified at 300 Pascals (0.044 psi) across the filter diameter.
Specifications used by the European Union: European Standard EN 1822-1:2009 defines categories of HEPA filters by their retention at a given most penetrating particle size (MPPS).
According to GMP, filters must be leak-free. This is verified by qualification and periodic leak testing according to ISO 14644-3. Recommended Practices for HEPA Filter Construction, Performance, Labeling, and Certification are maintained by the Institute of Environmental Sciences and Underwriters Laboratories (UL). Key requirements include:
■ IEST-RP-C021 “Testing HEPA and ULPA Media, which governs requirements for filter media
■ IEST-RP-CC001 “High Efficiency Particulate Air Filters and Ultraparticulate Air Filters”, governs overall filter construction and labeling requirements
■ IEST-RP-C034 “HEPA and ULPA Filter Leak Testing, for HEPA and UL PA filter penetration (leakage) testing
■ Testing and certification to UL900 flammability requirements
■ American Standard MIL-STD-282
Other testing standards include:
■ ISO 14644-3 Test Method – Qualified Persons Used During Installed Filter Leakage Testing
■ ISO 29463 (2017) – High Efficiency Filters and Filter Media for Removal of Airborne Particles
■ ISO 16890 (2017) – Air Filters for General Ventilation
■ PIC/s PI 032-2 GMP Guide
■ EN1822 used by HEPA/UPA filter manufacturers
High efficiency filter testing is a key technology and an important step to ensure a safe working environment and improve air quality. Filter test data is more effective in formulating a more scientific maintenance and replacement plan for customers.
KLC high efficiency filter testing program:
1. Smoke test
During the testing process, we use advanced instruments and technology to accurately measure the filter's capture rate of particles of different sizes and control a variety of environmental variables.

2. MPPS test
Comprehensively analyze the filter's performance under high flow conditions to ensure it can effectively maintain good air quality and system efficiency in actual use.

3. DOP Test
Used to verify the performance of filters in capturing fine particles of dirt, especially in fields such as pharmaceuticals, electronics and aviation where air quality is extremely high. This test uses DOP aerosol to accurately simulate the filtering effect of fine particles in actual working environments.

From October 23rd to 25th, KLC will appear at BIO EXPO 2024 with innovative filters and clean room equipment, participating in the grand event with biotechnology and clean room elites from around the world. This event not only provided KLC with a platform to showcase its innovative achievements, but also opened a new chapter of in-depth exchanges and cooperation with Turkish and even international counterparts.

This new concept of “Life Sciences 2024 Exhibitions of Turkey” includes 4 industries and 4 special exhibitions in one hand: Analytech 2024, Cleanroom 2024, Pharmanext 2024 and Biotecnica 2024 Exhibitions organized at the same time, in the same venue in order to create “ synergy” and “absolute concentration”.

At the exhibition, KLC's booth attracted the attention of many visitors. We showcased a variety of cutting-edge filters and clean equipment, covering solutions for multiple industries such as medical, pharmaceutical, food and electronics. Our products are known for their efficient filtration performance and excellent clean room control systems, winning high recognition from customers.
This exhibition provides a good platform for KLC to interact with industry partners and potential customers. Our professional team conducted in-depth technical exchanges with participants and shared the latest market trends, product innovations and visions for future development. Attendees showed strong interest in our high-efficiency filtration solutions and provided many case discussions on specific applications.



This exhibition provides KLC with a platform to showcase its strength and seek cooperation opportunities. In the future, we will continue to focus on developing more efficient and energy-saving filters and cleaning equipment to provide customers with first-class solutions. We look forward to working with all industry partners to jointly promote the development of clean technology.
Thank you to all friends who visited the KLC booth at the BIOEXPO exhibition. We look forward to establishing closer contact with you in the days to come and creating a better tomorrow in the clean field!
On April 19, the CPHI Japan 2024 came to a successful conclusion in Tokyo. CPHI JAPAN 2024 hold at Tokyo Big Sight, from April 17th to 19th. KLC showed up with clean room solutions and discussed new development trends and future opportunities in the pharmaceutical industry with experts and audiences.

During the exhibition, KLC displayed a complete set of pharmaceutical clean room equipment and filter products, attracting the attention of many attendees. Participants had an in-depth understanding of product features and application scenarios through communication with KLC representatives.
Going global in 2024 has allowed KLC to increase its visibility and influence in the pharmaceutical industry. KLC promises to continue to be committed to the innovation and development of indoor air solutions, provide customers with better services, and make greater contributions to environmental protection. Looking forward to our next encounter!
In the use of dust-free workshop, in addition to understanding the management system of dust-free workshop, we must also know how to control the humidity of dust-free workshop. The temperature and humidity of dust-free workshop are mainly determined according to process requirements, but under the condition of meeting process requirements, people's comfort should be considered. Therefore, we need to take effective solutions to the situation of high humidity in dust-free workshop.
How to reduce the high humidity in the dust-free workshop:
High humidity actually reduces the accumulation of static charge on the surface of the dust-free workshop and clean room. Lower humidity is more suitable for the accumulation of charge and becomes a potentially destructive source of static discharge. When the relative humidity exceeds 50%, the static charge begins to dissipate rapidly, but when the relative humidity is less than 30%, it can persist for a long time on an insulator or ungrounded surface. Therefore, in an environment with high relative humidity, the capillary force of concentrated water forms a connection bond between the particles and the surface, which can increase the adhesion of the particles to the siliceous surface. It is not important when the relative humidity is less than 50%, but when the relative humidity is around 70%, it becomes the main force for adhesion between particles.

Solutions to high humidity in dust-free workshops:
1. Increase dehumidification and drying: Add a dehumidifier in the clean workshop to increase the dryness of the workshop, and add it in the air conditioning duct during installation.
2. Seal equipment: Seal the equipment that may be affected as much as possible to reduce the impact of the humid environment in the workshop to achieve the purpose of safe storage.
3. Maintain ventilation: Ventilation can form convection of air inside and outside the workshop. When the temperature difference between inside and outside the workshop is greater, the air will flow faster, and the dehumidification effect on the workshop will naturally be more significant.
4. Moisture absorption in the workshop: In the rainy season or rainy days, when the humidity in the workshop is too high and it is not suitable for product storage, and the humidity outside the workshop is too high, it is not suitable for ventilation and moisture dissipation. You can use moisture absorption in the sealed warehouse to reduce the humidity in the warehouse.

In view of the problem of excessive humidity in the dust-free workshop, the use of a comprehensive solution can effectively improve the control ability of the workshop environment. Strengthening ventilation, using dehumidification equipment, controlling temperature, managing materials and strengthening monitoring are all important measures to ensure the stability of humidity in the dust-free workshop. Through these methods, the smooth progress of the production process can be ensured and the quality and safety of the products can be maintained.
From May 28th to 30th, the three-day SEMICON Southeast Asia 2024 was held in the Malaysia International Trade and Exhibition Center (MITEC)! KLC has been deeply involved in the clean industry for more than ten years. It appeared at SEMICON Southeast Asia 2024 to explore new trends and opportunities in the semiconductor industry with colleagues from the global industry.
2024 SEMICON Malaysia International Semiconductor Exhibition
SEMICON Southeast Asia is the largest semiconductor equipment exhibition in Southeast Asia. Attendees are offered the opportunity to discover new technology trends and markets, with a focus on key technologies and regional markets, where exhibitors can collaborate with new suppliers and discover new solutions. The purpose of this exhibition is to promote semiconductor technology through technology, innovation, and design.
At this event, KLC displayed brand-new filters, transfer windows and other equipment products. Visitors stopped at the booth to learn more about KLC clean systems. At the same time, KLC had in-depth exchanges with industry experts and companies from all over the world, and jointly discussed the needs and trends of clean rooms in the semiconductor industry, helping us to continuously improve and innovate products and services.

This exhibition was a very meaningful and valuable experience. We thank all participants and organizers for giving us the opportunity to demonstrate our expertise and technical strength. We hope to work with you in the future to jointly promote the development and progress of semiconductor clean production space and create a better production environment!
Dear customers,
We sincerely invite you to visit KLC's booth! KLC will participate in the Cleanroom Exhibition held in Türkiye from October 23 to 25, 2024.At this event, you will have the opportunity to experience our latest cleanroom technologies and solutions. During BIOEXPO, ANALYTECH,BIOTECNICA, and PHARMANEXT Exhibition will be held simultaneously, where you can have in-depth exchanges with industry experts and explore opportunities for future cooperation.
Our Booth: Rumeli 1 Hall 101/B
Time: October 23 - 25, 2024
Location: Istanbul Lutfi Kirdar Exhibition Halls Rumeli 1 Hall
We will be showcasing the latest products and technologies at the exhibition, including cleanroom design, equipment and materials, as well as our rich experience in the industry. Looking forward to seeing you at the exhibition and working together to create a better future.

Best wishes,
KLC Team
Present status of air filtration system in domestic pig farms
At present, domestic large-scale boar stations and original breeding pig farms are basically equipped with air filtration systems. Based on the fact that pig farm air filtration systems can keep the incidence of pigs in high-risk areas low, the industry has begun to pay attention to the air filtration epidemic prevention system.
Transmission routes of pathogens in animal husbandry
The main transmission routes are inter-field transmission and intra-field transmission. Almost all pathogens can be transmitted between fields through aerosols, mainly considering the pathogen load and meteorological conditions. As long as aerosols can be formed and are infectious, inter-field aerosol transmission can occur. Infection prevention is mainly to prevent inter-field transmission. Non-diffusion and detection and elimination are mainly to prevent intra-field transmission.
Viruses are transmitted in the form of vectors
Usually, the diameter of bioaerosol and dust particles is 0.3~5.0μm
SIV (swine influenza virus): 0.08μm-0.12μm
PRRSV (blue ear disease): 0.05μm-0.065μm
FMDV (oral disease virus): 0.022μm-0.03μm
PCV2 (porcine circovirus type I): 0.017μm-0.022μm
PRV (pseudorabies): 0.15μm-0.198μm
African swine fever: 0.175μm-0.2150μm
As can be seen from the above, the diameter of viruses is very small, but in general, viruses and bacterial pathogens can only be transmitted by attaching to carriers, mainly in the form of bioaerosols. The diameter of common dust particles or bioaerosols in nature is usually 0.3μm~5.0μm. Air filters can filter virus carriers, thereby playing a role in virus filtering.
Filtration principle
Air filters do not directly filter viruses or bacteria. What they actually filter are the transmission media of pathogens, namely dust particles or other aerosols. Pathogens themselves cannot spread through autonomous flight and need to be attached to a medium to spread. The diameter of this medium in nature is usually 0.3~1 micron, so air filters also filter these 0.3~1 micron particles to intercept bacteria-carrying particles.
At present, domestic large-scale pig farms are equipped with air filtration systems to filter particles attached with these viruses to reduce the risk of virus transmission.
Currently, common ventilation and filtration methods include negative pressure ventilation and filtration, positive pressure ventilation and filtration, and balanced ventilation and filtration. The choice of ventilation and filtration method depends on the level of air cleanliness required in the pig farm. At present, the negative pressure ventilation solution has good cooling effect and relatively economical energy consumption, which is adopted by most large-scale pig farms in China.
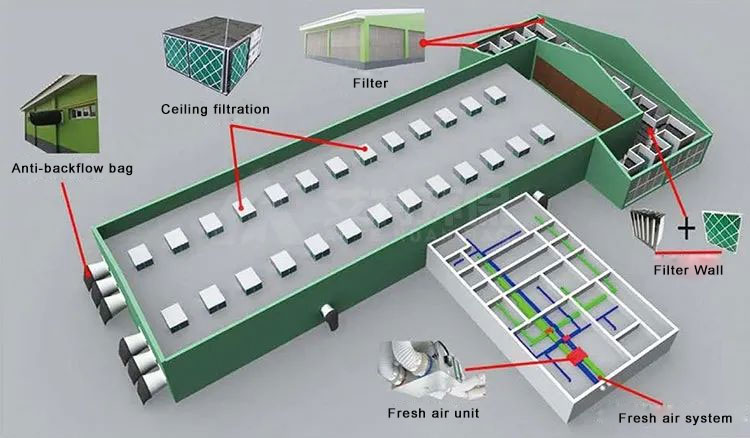
▶In the entire pig house air ventilation system, two or three layers of filters are installed on the outside of the air filter to clean the pig house production environment and isolate the invasion and cross infection of mosquitoes, flies and rats.
▶The boar house generally uses G4 coarse-effect filter + W-type high-efficiency air filter to form the main filter wall. The main filter wall blocks airborne pig farm pathogens, and the purification efficiency reaches L9. The long-term purification efficiency of 0.3μm aerosols or particles is greater than 95%.
▶The ceiling filter system is installed on the pig house ventilation window for ventilation of pig houses in low temperature seasons under negative pressure ventilation mode.
The commonly used functional room configurations for microbial testing in medical device production include: sterile room, microbial room and positive room. They are important facilities mainly used to ensure the sterile environment and microbial control in the production process of medical devices.
01. Functions of each laboratory
1) Sterile room: mainly used for resuscitation and propagation of bacterial strains, sterility testing of samples and other tests.
2) Microbial room: mainly provides a relatively clean environment for testing. The experimental content is mainly limit inspection, that is, testing the bacterial content in a specified amount of sample.
3) Positive room: mainly used for positive control, such as effect verification, bacterial species identification and other tests or tests that require the addition of bacteria. The samples in the positive room basically need to be added with bacteria (such as the bactericidal effect of the bactericidal agent, a certain amount of bacterial solution must be added to the bactericidal agent to verify the bactericidal effectiveness).
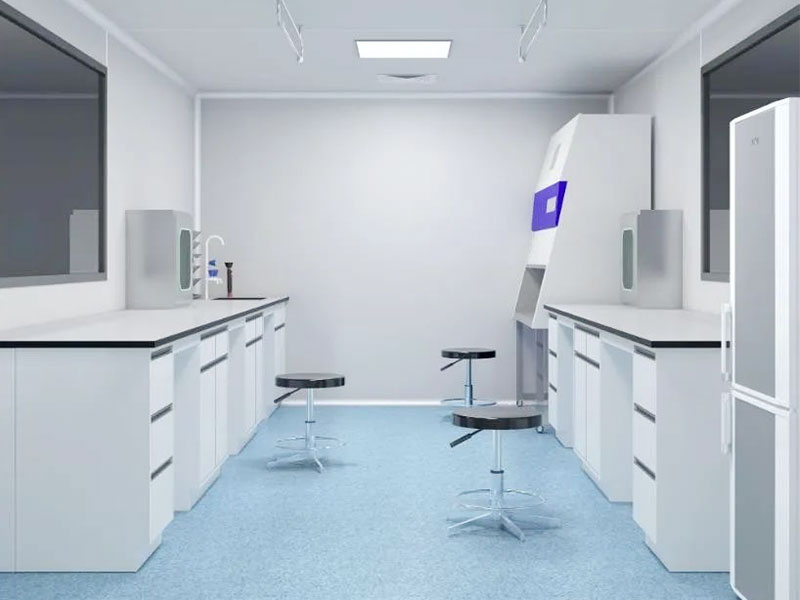
02. Laboratory requirements
Microbial room
(1) Cleanliness requirements: The cleanliness requirement of the microbial room is Class C.
(2) Pressure difference requirements: The static pressure difference between adjacent rooms with different positive pressure and air cleanliness levels should be greater than 5Pa, and the static pressure difference between the clean room and the outdoor atmosphere should be greater than 10Pa.
(3) Area requirements: Generally, it is an independent room of 4-10 square meters.
(4) Main equipment equipped: culture medium, incubator, microscope and other experimental equipment, clean bench (or isolator).
(5) Indoor temperature and humidity are controlled at 18-26℃, 40-60%.
Aseptic room:
(1) Cleanliness requirements: The cleanliness requirement of the aseptic room is Class C, and the cleanliness of the operation area must reach Class A or a clean bench of the same level should be placed.
(2) Pressure difference requirements: The static pressure difference between adjacent rooms with different positive pressure and air cleanliness levels should be greater than 5Pa, and the static pressure difference between the clean room and the outdoor atmosphere should be greater than 10Pa.
(3) Area requirements: Generally, it is an independent room of 4-10 square meters.
(4) Main equipment equipped: clean bench (or biological safety cabinet).
(5) Sterile laboratories need good lighting, avoid humidity, and be far away from toilets and contaminated areas. A buffer room should be set up outside the sterile room, with staggered door directions to prevent airflow from bringing in bacteria.
(6) Indoor temperature and humidity should be controlled at 18-26℃, 40-60%.
Positive room:
(1) Cleanliness requirements: The cleanliness requirement for the positive room is Class C
(2) Pressure difference requirements: The positive room is at negative pressure relative to the buffer room, generally ≥5Pa. The first shift should maintain positive pressure for changing shoes, the second shift should maintain a positive pressure of no less than 10Pa for the first shift, and the positive control room should maintain a relative negative pressure relative to the buffer room.
(3) Area requirements: Generally, it is an independent room of 4-10 square meters.
(4) Main equipment: biological safety cabinet (there are also vertical laminar flow workbenches).
(5) The first shift, second shift, buffer room, and positive control room can use a purification air supply system. This system is a fresh air system and the air cannot be recycled. Exhaust air can be discharged directly to the outside, but it must be filtered by high efficiency before being discharged to the outside.
(6) Indoor temperature and humidity are controlled at 18-26℃, 40-60%.

03. Other requirements
(1) Sterile laboratories, microbiological laboratories, and positive laboratories should be separated from each other. Because the samples in the microbiological room may contain bacteria, while the samples in the positive room basically need to be added with bacteria. Therefore, the microbiological room and the positive room cannot be mixed. If conditions permit, they need to be built separately. Otherwise, false positives will appear when doing limit tests in the positive room, and bacterial tests in the microbiological room will pollute the environment of the microbiological room.
(2) The microbiological room is not for sterile testing. Sterility testing should be performed in a separate sterile room.
(3) The laboratory should also have supporting preparation areas, culture areas, disinfection areas and other auxiliary rooms.
(4) Whether it is a microbiological room, a positive room or a sterile room, the common characteristics of these rooms are that the floors and walls are smooth and hard after decoration, and the instruments and equipment are simply arranged, which is easy to clean.
(5) Microbiological rooms, positive rooms and sterile rooms must take necessary disinfection measures to ensure that the laboratory clean conditions are qualified, such as setting the ultraviolet sterilization lamp to 2~2.5W/square meter.
(6) The laboratory should provide sufficient illumination according to production requirements. The illumination value should not be lower than 300LX.

The construction of the laboratory must follow the principle of coexistence of safety and economy, especially the construction of biosafety experimental sites, which should be considered from the perspective of biosafety and the possible impact of cross-contamination on experimental results, to ensure that the unidirectional air flow area, work surface and internal environment of each area meet the standards.
The purpose of controlling the pressure difference of each clean room in a pharmaceutical factory is to ensure that when the clean room is working normally or the balance is temporarily disrupted, the air can flow from the area with high cleanliness to the area with low cleanliness, so that the cleanliness of the clean room is not disturbed by polluted air. Clean room pressure difference control is an important part of the design of the purification air conditioning system of the clean room of a pharmaceutical factory, and it is an important measure to ensure the cleanliness of the clean area. The clean room pressure difference control chapter of the "Clean Room Design Specification" GB50073-2013 (hereinafter referred to as the "Clean Specification") includes 5 contents, all of which are clauses for clean room pressure difference control. Article 16 of the "Good Manufacturing Practice for Pharmaceuticals" (revised in 2010) requires that the clean area must have a device to indicate the pressure difference.
Clean room pressure difference control is divided into 3 steps:
The first step is to determine the pressure difference of each clean room in the clean area;
The second step is to calculate the pressure difference air volume of each clean room in the clean area to maintain the pressure difference;
The third step is to take technical measures to ensure the pressure difference air volume of the clean room and maintain the constant pressure difference of the clean room.
1. Determine the pressure difference of each clean room in the clean area
According to the requirements of Article 6.2.1 and Article 6.2.2 of the "Clean Specification", a certain pressure difference must be maintained between the clean room and the surrounding space, and the positive or negative pressure difference should be maintained according to the production process requirements. The pressure difference between clean rooms of different levels and between clean areas and non-clean areas should not be less than 5 Pa, and the pressure difference between the clean area and the outdoors should not be less than 10 Pa.
① Pressure difference of each clean room in the same clean area
In actual engineering, to determine the pressure difference of each clean room in the same clean area, the pressure of each clean room can be compared with the clean area corridor, with the pressure value of the clean area corridor as the benchmark. Because the clean area corridor runs through each clean room, the pressure difference between each clean room and the clean area corridor is determined, and the pressure difference between the clean rooms is also determined. The pressure values of all clean rooms are based on the pressure value of the clean area corridor, so there will be no confusion between the pressure difference values. For example, in a solid preparation workshop, the positive pressure value of the clean area corridor can be determined to be 18 Pa (0 Pa outside the clean area); the crushing room and weighing room have serious dust dispersion, and are generally connected to the clean area corridor through the antechamber. In order to prevent the airflow with high dust content in the room from spreading to other rooms through the corridor, the positive pressure value of the crushing room and weighing room can be determined to be 12 Pa, and the positive pressure value of the antechamber can be determined to be 15 Pa. In this way, the crushing room and weighing room are at negative pressure relative to the antechamber, and the antechamber is at negative pressure relative to the clean area corridor. The airflow flows from the clean area corridor to the antechamber, and from the antechamber to the crushing room and weighing room. The clean and dry equipment storage room is used to store washed and dried equipment. To avoid contamination, the positive pressure value of the room can be determined to be 21 Pa to prevent the airflow from the corridor from flowing into the room.
② Pressure difference between clean areas of different levels
To determine the pressure difference between clean areas of different levels, you can first determine the positive pressure of the clean room with a low cleanliness level, and then increase the base of the positive pressure value in sequence to determine the positive pressure of the clean room with a high cleanliness level. For example, the water injection workshop contains a 100,000-level clean area, a 10,000-level clean area, and a partial 100-level clean area. The positive pressure value of the corridor of the 100,000-level clean area is 18 Pa, so it is necessary to increase the overall positive pressure value of the 10,000-level clean area. In short, there should be a positive pressure difference of no less than 5 Pa between the adjacent rooms of the 10,000-level clean area and the 100,000-level clean area. There are rooms in the 100-level clean area in the 100-level clean area. For this, just increase the positive pressure of the rooms in the 100-level clean area.
③ Pressure difference in clean areas in special cases
Some pharmaceutical production workshops, such as soft capsule production workshops, have clean rooms with different relative humidity in the same clean area. For this, the relatively dry clean room should be controlled to be positively pressurized relative to the adjacent clean room to prevent wet air from flowing into the dry clean room. The production plant of highly allergenic drugs such as penicillin, and clean rooms with drug powder exposure such as filling rooms should maintain a relative negative pressure.
The purpose of controlling the pressure difference of each clean room in a pharmaceutical factory is to ensure that when the clean room is working normally or the balance is temporarily disrupted, the air can flow from the area with high cleanliness to the area with low cleanliness, so that the cleanliness of the clean room is not disturbed by polluted air. Clean room pressure difference control is an important part of the design of the purification air conditioning system of the clean room of a pharmaceutical factory, and it is an important measure to ensure the cleanliness of the clean area. The clean room pressure difference control chapter of the "Clean Room Design Specification" GB50073-2001 (hereinafter referred to as the "Clean Specification") includes 5 contents, all of which are clauses for clean room pressure difference control. Article 16 of the "Good Manufacturing Practice for Pharmaceuticals" (revised in 1998) requires that the clean area must have a device to indicate the pressure difference.

2. Determine the pressure differential air volume to maintain the pressure difference
The pressure differential air volume to maintain the positive pressure difference in each clean room in the clean area needs to be supplemented by outdoor fresh air. Therefore, the size of the positive pressure differential air volume in the clean room directly affects the fresh air ratio of the purification air conditioning system and the energy consumption of the purification air conditioning system. The pressure differential air volume to maintain the negative pressure difference in each clean room in the clean area penetrates into the clean room from the outside of the clean room. In many cases, it is the outdoor air that has not been purified. Therefore, the size of the negative pressure differential air volume in the clean room is directly related to the cleanliness of the negative pressure clean room. At present, the common methods for calculating the pressure differential air volume in the clean room are the gap method and the ventilation number method. The gap method is to estimate the pressure differential air volume of the clean room based on the total length of the gaps such as doors and windows in the clean room. However, in actual applications, the work of counting the number of gaps such as doors and windows is relatively cumbersome and prone to errors and omissions, and is currently less used. The ventilation number method is to estimate the pressure differential air volume of the clean room based on the number of ventilation times in the clean room. In actual engineering applications, this method has the advantages of simplicity, ease of operation, and high accuracy, and is a commonly used method. Article 6.2.3 of the Clean Specifications recommends the ventilation frequency method and proposes to select according to the following data: 1 to 2 times/hour when the pressure difference is 5 Pa, 2 to 4 times/hour when the pressure difference is 10 Pa. Other reference books also have recommended values, such as the Practical Heating and Air Conditioning Design Manual (hereinafter referred to as the Manual), which recommends 0.7 times/hour when the pressure difference is 4.9 Pa, and 1.2 times/hour when the pressure difference is 9.81 Pa. However, in actual applications, people have found that the data recommended by the Clean Specifications tend to be conservative, consume a large amount of pressure difference air volume, and are not economical; while the values recommended by the Manual are more appropriate. In actual projects, it is entirely possible to reduce the pressure difference air volume in the room by strengthening the air tightness of the clean room enclosure structure. According to the size of the pressure difference value of the clean room, the pressure difference should be selected according to the ventilation frequency of 1 to 2 times/hour.
The purpose of controlling the pressure difference of each clean room in a pharmaceutical factory is to ensure that when the clean room is working normally or the balance is temporarily disrupted, the air can flow from the area with high cleanliness to the area with low cleanliness, so that the cleanliness of the clean room is not disturbed by polluted air. Clean room pressure difference control is an important part of the design of the purification air conditioning system of the clean room of a pharmaceutical factory, and it is an important measure to ensure the cleanliness of the clean area. The clean room pressure difference control chapter of the "Clean Room Design Specification" GB50073-2001 (hereinafter referred to as the "Clean Specification") includes 5 contents, all of which are clauses for clean room pressure difference control. Article 16 of the "Good Manufacturing Practice for Pharmaceuticals" (revised in 1998) requires that the clean area must have a device to indicate the pressure difference.
3. Maintain constant pressure difference in clean rooms
The above-mentioned pressure difference value and pressure difference air volume in clean rooms are only theoretical values, which need to be realized by certain technical measures and facilities. In actual projects, there are many ways to control the pressure difference in clean rooms:
Under normal circumstances, there are many ways to adopt a constant air volume system, that is, first ensure that the clean room air supply volume is relatively constant, adjust the clean room return air volume or exhaust air volume, so as to control the clean room pressure difference air volume and maintain the clean room pressure difference value; you can also install a manual split multi-leaf regulating valve or butterfly valve on the clean room return (exhaust) air branch pipe to adjust the return (exhaust) air volume and control the indoor pressure difference. Its advantages are simple equipment and effectiveness.
The method of adjusting the pressure difference in the clean room during the commissioning of the air conditioning system has the disadvantage that when the pressure difference in the clean room deviates from the set value during the operation of the air conditioning system, it is more troublesome to adjust. This method is used in conjunction with other methods and is one of the common means of controlling the pressure difference in clean rooms in current projects.
Installing a damping layer (such as a single-layer non-woven fabric, stainless steel filter, aluminum alloy filter, nylon filter, etc.) at the return (exhaust) air outlet of the clean room can effectively ensure the positive pressure of the clean room, but the filter as the damping layer needs to be replaced frequently to prevent the positive pressure in the clean room from being too high.
Install a residual pressure valve on the partition wall of the adjacent room to control the positive pressure. Its advantages are simple and reliable equipment, and its disadvantages are that the residual pressure valve is relatively large in size, the ventilation volume is limited, it is not convenient to install, and it is not convenient to connect with the air duct, and it can only be installed in individual clean rooms.
Install an electric actuator system on the valve shaft of the return (exhaust) air branch regulating valve in the clean room, so as to form an electric regulating valve with the corresponding valve. According to the feedback of the clean room pressure difference value, fine-tune the valve opening, and automatically adjust the pressure difference in the clean room to return to the set value. This method is used to control the pressure difference in the clean room more reliably and accurately, and the control system cost is not high. It is widely used in engineering practice. The system can be installed on the return (exhaust) air branch regulating valve of the clean room or the typical clean room that needs to display the pressure difference.
Install Venturi air volume control valves on the air supply branch and return (exhaust) branch in the clean room. There are three types of Venturi valves: fixed air volume valves, which can provide stable air flow; bistable valves, which can provide two different air flow rates, namely maximum and minimum flow rates; variable air volume valves, which can control the air flow rate through closed-loop response to instructions and flow feedback signals in less than 1 second. Venturi valves are not affected by changes in duct pressure, have rapid responses (less than 1 second), and are precisely adjusted, but the equipment is relatively expensive and is suitable for use in some biological product production plants that require negative pressure control, toxic and biosafety laboratories (such as P3 biological laboratories), and other places. Because personal safety issues must be considered, the system pressure differential control must be high-precision and highly reliable. In this regard, by using constant air volume valves and bistable valves, the supply and exhaust air volumes of the clean room (or laboratory) can be strictly controlled, thereby forming a stable pressure differential air volume and controlling the pressure differential of the clean room (or laboratory) to be stable; using variable air volume valves to regulate the room so that the flow rate of the supply air duct valve tracks the flow rate of the exhaust air duct valve, a stable pressure differential air volume can be formed and the pressure differential of the clean room (or laboratory) can be controlled to be stable.
We will exhibit at MEGA CLIMA NIGERIA 2024 from May 21st to 24th.
We believe that your participation will add more value to the exhibition. We sincerely invite you to participate in the exhibition and discuss the development trends and future opportunities of clean rooms in the refrigeration field. If you need more information or have any questions, please feel free to contact us.
BOOTH: MEGA CLIMA #B07
Address: Landmark Centre, Lagos-NIGERIA
Time: May 21-23, 2024
Thanks!
Sincerely

- Automotive Engine Rubber Parts8
- Automotive Lamps Rubber Parts5
- Automotive Suspension Rubber Parts2
- Automotive Wiring Harness Rubber Parts3
- Extrusion Sealing Strip1
- Industrial Electrical Rubber Parts3
- Industrial Scanners2
- Industrial electrical control3
- Industrial slings4
- Machine Tool Blades1
- Membrane Products1
- Motor1
- Racecource Rubber Products3
- Rubber Forklift Attachments1
- Rubber and plastic Parts1
- Seal2
- Tubular Motor2
- industrial hose1
- mold1
- plc2
- pump1
- racking2